Abstract
Introduction: Hemophagocytic lymphohistiocytosis (HLH) is a life threatening immune dysregulation disorder. Pathophysiologically, HLH is characterized by T and NK cell cytotoxic defects leading to overwhelming immune activation which drive disease progression. T and NK cell abnormalities have been the focus of research in this disorder. Yet, little is known about the effect of immune activation and inflammation on B cell development and maturation. Previous work by Sumegi et al. (2001) using mRNA expression array from peripheral blood mononuclear cells of HLH patients showed marked down regulation of genes involved in B cell differentiation. Interestingly, hypogammaglobulinemia and common variable immune deficiency-like presentation is described in patients with primary HLH specifically in STXBP2, UNC13-D, and XIAP deficiency. In addition to defective T and NK cells, B cell defects and hypogammaglobulinemia could predispose patients to infections. Thus, evaluating the effect of HLH on B cell development and function is critical for the characterization of the disease which may translate into improvements in clinical management and possibly outcome. To further gain such insight, we analyzed the flow cytometric profiles from bone marrows of patients with a diagnosis of HLH along with other clinical and laboratory data.
Methods: A total of 39 patients with a diagnosis of HLH from April 2009 to February 2017 were categorized into groups of Primary (8 patients with a confirmed genetic mutation) and Secondary [19 patients with HLH triggered by infection and 12 patients with macrophage activation syndrome (MAS)]. Five bone marrow samples from healthy controls were concurrently evaluated. All flow cytometric profiles were obtained from bone marrow aspirates and were analyzed using the BDFACS DIVATM Software (BD Biosciences, San Jose, CA). Known specific biomarkers for lymphoid and myeloid characterization were used to illustrate the cell populations. Chart review was performed using EPIC (EpicSystems, Verona, WI) to collect patient demographic data, corresponding laboratory values around the time of bone marrow sampling, treatment, infectious history, underlying disease, and survival.
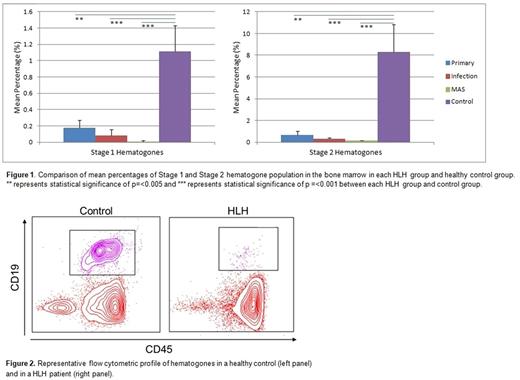
Results: Stage 1 and Stage 2 hematogones (which represent early B cell development in the bone marrow) were decreased by 12 fold (0.09 ± 0.05% vs. 1.12 ± 0.31%, p=<0.001) and 23 fold (0.36 ± 0.17% vs. 8.31 ± 2.52, p=<0.001) respectively in HLH patients when compared to healthy controls. Interestingly, further evaluation within different HLH subtypes revealed that the decrease in Stage 1 and 2 hematogones were more marked in patients with MAS when compared to children with primary HLH (Stage 1: Primary 0.18 ± 0.09%, Infection-triggered 0.08 ± 0.04%, MAS 0.01 ± 0.01%, Control 1.12 ± 0.31%; Stage 2: Primary 0.67 ± 0.35%, Infection-triggered 0.29 ± 0.13%, MAS 0.13 ± 0.04%, Control 8.31 ± 2.52%) (Figures 1 and 2). These findings suggest that early B cell development is affected during active HLH. Of the 11 HLH patients who had lymphocyte subsets checked prior to receiving steroid treatment, 45% had low absolute B cell levels for their age. Of the 19 HLH patients who had an IgG level checked prior to receiving immunoglobulin treatment, 21% had low levels for their age. As expected, the activated T cell population (CD38+ CD19-) was significantly higher in the HLH groups compared to the control group (Primary 63.4 ± 6.95%, p=0.021; Infection-triggered 66.6 ± 3.2%, p=<0.001; MAS 65.2 ± 5.36%, p=0.008; p values represent each HLH group vs. Control 37.9 ± 4.28%).
Conclusion: Our analysis of bone marrow B cell development highlights marked suppression of early B cell development in patients with active HLH. In addition, a significant proportion of these patients have low B cell numbers for age and hypogammaglobulinemia. We plan to further investigate longitudinal data from subsequent bone marrow samples obtained from a subset of patients to investigate the effect of persistent inflammation on mature B cells. Furthermore, studies evaluating the effect of HLH-driven inflammation on peripheral B cell memory and class switching are being pursued.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal